Avogadro's Number Is Equal To 6.02x1023
catanddoghelp
Dec 01, 2025 · 13 min read
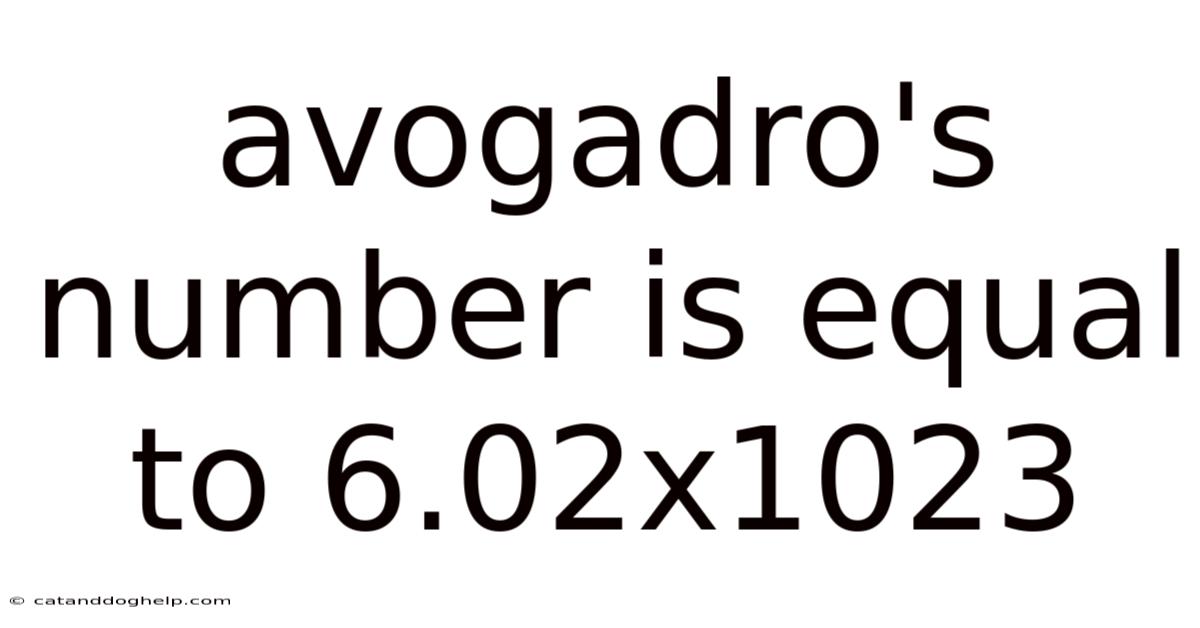
Table of Contents
Imagine trying to count every grain of sand on a beach, or every star in the night sky. Daunting, isn't it? Now imagine needing to count something even smaller, like atoms or molecules. That's where Avogadro's number comes in. It's not just a random number; it's a cornerstone of chemistry, a bridge between the macroscopic world we can see and the microscopic world of atoms and molecules. Without it, much of modern chemistry simply wouldn't be possible.
Think of baking a cake. You follow a recipe that calls for specific amounts of ingredients – 2 cups of flour, 1 cup of sugar, and so on. These measurements allow you to consistently recreate the cake. In chemistry, we also need a way to "measure" ingredients – not in cups or grams, but in numbers of atoms or molecules. Avogadro's number, 6.02 x 10^23, provides us with that essential measuring tool, enabling chemists to perform calculations and conduct experiments with precision and accuracy.
Main Subheading
Avogadro's number, universally recognized as 6.02 x 10^23, represents the number of constituent particles – typically atoms, molecules, or ions – that are contained in one mole of a substance. This seemingly arbitrary number is, in fact, the result of meticulous scientific investigation and plays a crucial role in connecting the atomic scale to the macroscopic scale. Understanding its significance requires delving into the history of chemistry, the development of the concept of the mole, and the experimental methods used to determine its value.
The concept of the mole emerged from the need to quantify chemical reactions. Early chemists recognized that elements combine in fixed proportions by mass. This led to the development of the concept of atomic weights. However, knowing the relative weights of atoms wasn't enough. Chemists needed a way to relate these weights to the actual number of atoms involved in a reaction. This is where the idea of a "unit" of atoms – a specific number that would always correspond to the same relative weight – became essential.
Comprehensive Overview
Definition and Significance
Avogadro's number (often denoted as NA) is defined as the number of carbon-12 atoms in 12 grams of carbon-12. This definition ties the number directly to the atomic mass scale, providing a consistent and reproducible standard. The mole, as a unit, is analogous to other counting units like a dozen (12) or a gross (144). However, the mole represents a vastly larger quantity, reflecting the immense number of atoms and molecules present even in small amounts of matter.
The significance of Avogadro's number lies in its ability to bridge the gap between the microscopic and macroscopic worlds. It allows chemists to:
- Relate mass to the number of atoms/molecules: Knowing the molar mass of a substance (the mass of one mole) and Avogadro's number, one can calculate the number of atoms or molecules in a given mass of that substance.
- Determine empirical and molecular formulas: By experimentally determining the mass composition of a compound, chemists can use Avogadro's number to calculate the number of moles of each element present and, consequently, the empirical formula.
- Perform stoichiometric calculations: Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. Avogadro's number is fundamental to stoichiometric calculations, allowing chemists to predict the amount of reactants needed or products formed in a given reaction.
- Calculate gas volumes: The ideal gas law, PV = nRT, relates pressure (P), volume (V), number of moles (n), the ideal gas constant (R), and temperature (T). Avogadro's number is essential for converting between the number of molecules and the number of moles in gas calculations.
Historical Context
The number is named after Italian scientist Amedeo Avogadro, though he did not actually determine the value himself. Avogadro proposed in 1811 that equal volumes of gases at the same temperature and pressure contain the same number of molecules, regardless of their chemical nature. This hypothesis, now known as Avogadro's Law, laid the groundwork for understanding the relationship between the number of particles and the macroscopic properties of gases.
However, it wasn't until long after Avogadro's death that scientists were able to accurately determine the number of particles in a mole. Early attempts to estimate the number relied on various physical measurements, such as the behavior of gases, the properties of solutions, and the characteristics of blackbody radiation. These early estimates varied widely, but they gradually converged toward the modern value.
Methods of Determination
Several experimental methods have been used to determine Avogadro's number with increasing precision over the years. Some of the most notable methods include:
- Electrolysis: Electrolysis involves using an electric current to drive a non-spontaneous chemical reaction. By carefully measuring the amount of charge passed through an electrolytic cell and the amount of substance produced or consumed, one can calculate Avogadro's number. This method relies on the relationship between electric charge, the number of electrons, and the number of moles of substance.
- Brownian Motion: Brownian motion is the random movement of particles suspended in a fluid (liquid or gas). Albert Einstein developed a theoretical treatment of Brownian motion that related the mean squared displacement of the particles to Avogadro's number, the gas constant, and temperature. By carefully observing and measuring Brownian motion, scientists could estimate Avogadro's number.
- X-ray Diffraction: X-ray diffraction is a technique used to determine the atomic and molecular structure of crystalline materials. By shining X-rays onto a crystal and analyzing the diffraction pattern, scientists can determine the size and arrangement of atoms in the crystal lattice. Knowing the volume of the unit cell (the smallest repeating unit in the crystal) and the molar mass of the substance, one can calculate Avogadro's number.
- The Oil Drop Experiment: Robert Millikan's oil drop experiment, primarily designed to determine the charge of a single electron, also provided a means to calculate Avogadro's number. Knowing the charge of a single electron and the Faraday constant (the charge of one mole of electrons), Avogadro's number can be calculated as the ratio of the Faraday constant to the elementary charge.
- Atomic Mass and Molar Mass: The most accurate modern determination of Avogadro's number relies on precisely measuring the atomic mass of silicon-28 and the molar mass of a highly purified silicon crystal. Using X-ray interferometry, the volume of the unit cell of the silicon crystal can be determined with extreme accuracy. Combining these measurements allows for a precise calculation of Avogadro's number.
The Mole and Molar Mass
The mole is the SI unit for the "amount of substance." It is defined as the amount of substance containing as many elementary entities (atoms, molecules, ions, etc.) as there are atoms in 12 grams of carbon-12. Thus, by definition, the number of entities in one mole is Avogadro's number.
Molar mass, on the other hand, is the mass of one mole of a substance. It is typically expressed in grams per mole (g/mol). The molar mass of an element is numerically equal to its atomic mass in atomic mass units (amu). For example, the atomic mass of carbon is approximately 12 amu, and the molar mass of carbon is approximately 12 g/mol. For compounds, the molar mass is the sum of the atomic masses of all the atoms in the chemical formula.
The relationship between mass, moles, and molar mass is given by the following equation:
n = m / M
Where:
- n is the number of moles
- m is the mass of the substance
- M is the molar mass of the substance
This equation is fundamental to many calculations in chemistry and is used extensively in stoichiometric problems.
Practical Applications
The implications of Avogadro's number extend far beyond theoretical calculations. It is a practical tool used in various fields, including:
- Pharmaceuticals: In drug development and manufacturing, accurate measurements of chemical quantities are crucial for ensuring the safety and efficacy of medications. Avogadro's number is used to calculate the precise amount of active ingredients needed in a formulation and to ensure consistency between batches.
- Materials Science: In materials science, Avogadro's number is used to determine the composition and structure of materials at the atomic level. This knowledge is essential for designing and synthesizing new materials with desired properties.
- Environmental Science: In environmental monitoring, Avogadro's number is used to quantify pollutants and contaminants in air, water, and soil. This information is crucial for assessing environmental risks and developing strategies for remediation.
- Nanotechnology: In nanotechnology, Avogadro's number is used to manipulate and control matter at the nanoscale. This allows for the creation of new devices and materials with unique properties.
Trends and Latest Developments
The determination of Avogadro's number continues to be an area of active research. While the currently accepted value is known with very high precision, scientists are constantly seeking to improve the accuracy and reliability of its determination. One emerging trend is the use of advanced techniques, such as quantum metrology and single-atom counting, to determine Avogadro's number with even greater precision.
Furthermore, there is growing interest in using Avogadro's number as a fundamental constant for redefining the kilogram, the SI unit of mass. Currently, the kilogram is defined by a physical artifact – the International Prototype Kilogram (IPK), a platinum-iridium cylinder kept at the International Bureau of Weights and Measures (BIPM) in France. However, the IPK is subject to changes in mass over time, which can lead to inconsistencies in measurements.
By defining the kilogram in terms of a fundamental constant like Avogadro's number, the mass standard would be based on an unchanging property of nature, ensuring greater stability and accuracy. This redefinition was officially adopted in 2019, marking a significant milestone in the history of metrology. This new definition links the kilogram to the Planck constant, which is related to energy and frequency, and ultimately to Avogadro's number through fundamental physical relationships.
Tips and Expert Advice
Understanding and applying Avogadro's number can be challenging for students and professionals alike. Here are some tips and expert advice for mastering this essential concept:
-
Understand the Definition: Make sure you have a clear understanding of what Avogadro's number represents – the number of constituent particles in one mole of a substance. Avoid simply memorizing the number; focus on understanding its significance.
-
Practice Mole Conversions: Practice converting between mass, moles, and the number of particles using Avogadro's number and molar mass. Work through numerous example problems to solidify your understanding of these conversions. A strong foundation in these conversions is crucial for success in stoichiometry and other areas of chemistry.
-
Pay Attention to Units: Always pay close attention to units when performing calculations involving Avogadro's number. Ensure that you are using consistent units for mass, moles, and molar mass. Incorrect units can lead to significant errors in your calculations. Double-check your units at each step of the calculation to avoid mistakes.
-
Visualize the Scale: Try to visualize the sheer magnitude of Avogadro's number. It's a truly enormous number, representing the vast number of atoms and molecules present even in seemingly small amounts of matter. Comparing it to other large numbers, like the number of stars in the galaxy, can help you appreciate its scale.
-
Use Dimensional Analysis: Dimensional analysis (also known as unit analysis) is a powerful technique for solving problems involving conversions and calculations. By carefully tracking units and ensuring that they cancel out correctly, you can avoid errors and ensure that your answer has the correct units.
-
Relate to Real-World Examples: Connect Avogadro's number to real-world examples to make the concept more tangible. For example, consider how Avogadro's number is used in the manufacturing of pharmaceuticals or in the analysis of environmental pollutants.
-
Master Stoichiometry: Stoichiometry is the application of Avogadro's number to quantitative chemical reactions. Mastering stoichiometry is crucial for understanding how chemical reactions occur and for predicting the amounts of reactants and products involved. Practice a wide variety of stoichiometry problems, including those involving limiting reactants, percent yield, and gas volumes.
-
Use Online Resources: Take advantage of the many online resources available for learning about Avogadro's number and stoichiometry. Many websites offer tutorials, practice problems, and interactive simulations that can help you master these concepts.
-
Seek Help When Needed: Don't hesitate to seek help from your instructor, classmates, or online forums if you are struggling with Avogadro's number. Chemistry can be a challenging subject, and it's important to get help when you need it.
-
Understand its Limitations: While Avogadro's number is incredibly useful, it's important to remember that it is based on certain assumptions and approximations. For example, it assumes that atoms and molecules are point masses and that they behave ideally. In reality, atoms and molecules have finite size and can interact with each other, which can lead to deviations from ideal behavior.
FAQ
Q: Why is Avogadro's number so large?
A: Avogadro's number is large because atoms and molecules are incredibly small. It takes a huge number of these tiny particles to make up a macroscopic amount of substance that we can see and measure.
Q: Is Avogadro's number a constant?
A: Yes, Avogadro's number is considered a fundamental constant of nature. Its value is fixed and does not depend on the substance being considered.
Q: What is the difference between Avogadro's number and the mole?
A: Avogadro's number is a specific number (6.02 x 10^23), while the mole is a unit of measurement that represents that number of entities. The mole is analogous to other counting units like a dozen or a gross.
Q: How is Avogadro's number used in chemistry?
A: Avogadro's number is used to relate mass to the number of atoms/molecules, determine empirical and molecular formulas, perform stoichiometric calculations, and calculate gas volumes. It's a fundamental tool for quantitative analysis in chemistry.
Q: What is the uncertainty in the value of Avogadro's number?
A: While the value of Avogadro's number is known with very high precision, there is still a small degree of uncertainty. The currently accepted value is 6.02214076 × 10^23 mol−1, with a standard uncertainty of about 0.00000001 × 10^23 mol−1.
Conclusion
Avogadro's number, 6.02 x 10^23, is more than just a number; it's a fundamental constant that bridges the microscopic world of atoms and molecules to the macroscopic world we experience. Its determination has been a triumph of scientific investigation, and its applications are widespread and essential to modern chemistry and related fields. From calculating the precise amounts of ingredients in pharmaceuticals to understanding the structure of new materials, Avogadro's number plays a crucial role. A firm grasp of this concept is essential for anyone studying or working in the chemical sciences.
Now that you have a deeper understanding of Avogadro's number, take the next step! Practice applying this knowledge to solve stoichiometry problems, explore online resources, and share this article with fellow students or colleagues. Deepening your understanding of these fundamental concepts ensures greater success in your scientific journey.
Latest Posts
Latest Posts
-
How To Do Divide With Decimals
Dec 01, 2025
-
How Many Oz In 1 Kilo
Dec 01, 2025
-
Words That Start With An A In Spanish
Dec 01, 2025
-
Avogadros Number Is Equal To 6 02x1023
Dec 01, 2025
-
How Many Meters Is 15 Ft
Dec 01, 2025
Related Post
Thank you for visiting our website which covers about Avogadro's Number Is Equal To 6.02x1023 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.